NEUROMUSCULAR DISORDERS & MUSCLE WASTING DISEASES
Building the wellness and clinical gold standards to measure muscle wasting and the impact of its treatment.
A Comprehensive, Painless, Portable Measure of Muscle Quality. Even @Home.
Our driving goal is to assess, monitor, and display changes in skeletal muscle composition and structure to aid in the diagnosis, prognosis, and treatment of neuromuscular and musculoskeletal diseases. We are deeply committed to help the dedicated clinicians, scientists, caregivers, and above all, patients who are battling these diseases, each and every day.
Neuromuscular Disorders are Growing.
Muscle Wasting is Skyrocketing.

- ALS will increase 69% by 2040
- Americans 65+ will reach 72M by 2030. Up to 30% will get sarcopenia
- Muscle wasting increases fall risk… #1 cause of elderly injury death
- 30M U.S. people projected to use GLP-1 obesity drugs by 2030 (JP Morgan).
Many Clinical Unmet Needs

No quantitative, comprehensive biomarker for measuring and monitoring neuromuscular disorders.

Current tools not sensitive and frequent enough to “see” early, single muscle changes.

“Diagnostic delay” can range 9-24 months from the time ALS symptoms first manifest.


MRI, now widely used to measure muscle conditions, is used infrequently, expensive, non-portable, and often traumatic.

Other muscle measures are invasive, in-clinic, and infrequent for monitoring or insensitive, not easily transportable, or not single muscle specific.

Clinicians are now seeking in-home measurements for better patient compliance, diversity, and more frequent data.




Quantitative
Provides quantitative data on functional capability while the muscle and subject remain entirely at rest.
portable
Portable non-invasive device that scans a muscle in a matter of seconds.
Painless
EIM engages painless, active monitoring.
comprehensive
One holistic measure of both muscle composition and infrastructure.
MYOLEX BY THE NUMBERS

Five breakthroughs help make the impossible… Possible
Quantitative & Comprehensive
![]() Quantitative device that non-invasively and comprehensively measures a single muscle’s full composition and micro architecture
Quantitative device that non-invasively and comprehensively measures a single muscle’s full composition and micro architecture
Aid Earlier ALS Diagnosis
![]() Highly sensitive, more frequently available, device that could assist clinical researchers in identifying ALS diseased muscles earlier**
Highly sensitive, more frequently available, device that could assist clinical researchers in identifying ALS diseased muscles earlier**
MRI Alternative for Muscles
![]() Alternative device that could replace* and/or augment magnetic resonance imaging (MRI) as the lead measurement device for upper and lower extremities, abdomen, shoulder, and hip muscles
Alternative device that could replace* and/or augment magnetic resonance imaging (MRI) as the lead measurement device for upper and lower extremities, abdomen, shoulder, and hip muscles
Smaller Clinical Trials
![]() Highly accurate, sensitive, and reproducible muscle condition assessment tool that enables pharmaceutical companies to conduct smaller, faster, more diverse clinical trials
Highly accurate, sensitive, and reproducible muscle condition assessment tool that enables pharmaceutical companies to conduct smaller, faster, more diverse clinical trials
Painless. Easy-to-Use. @Home
![]() Painless, portable, and easy-to-use muscle measurement device that remote subjects and their caregivers can use at home
Painless, portable, and easy-to-use muscle measurement device that remote subjects and their caregivers can use at home
*Requires FDA Approval.
**In mouse model research study, EIM identified ALS muscle deterioration very early at 8 weeks, four to six weeks earlier than other clinical standard muscle measures.
Why EIM is a Superior Muscle Assessment
Painlessly measures muscle composition and micro infrastructure
COMPREHENSIVE & HOLISTIC

MEASURES SINGLE MUSCLES
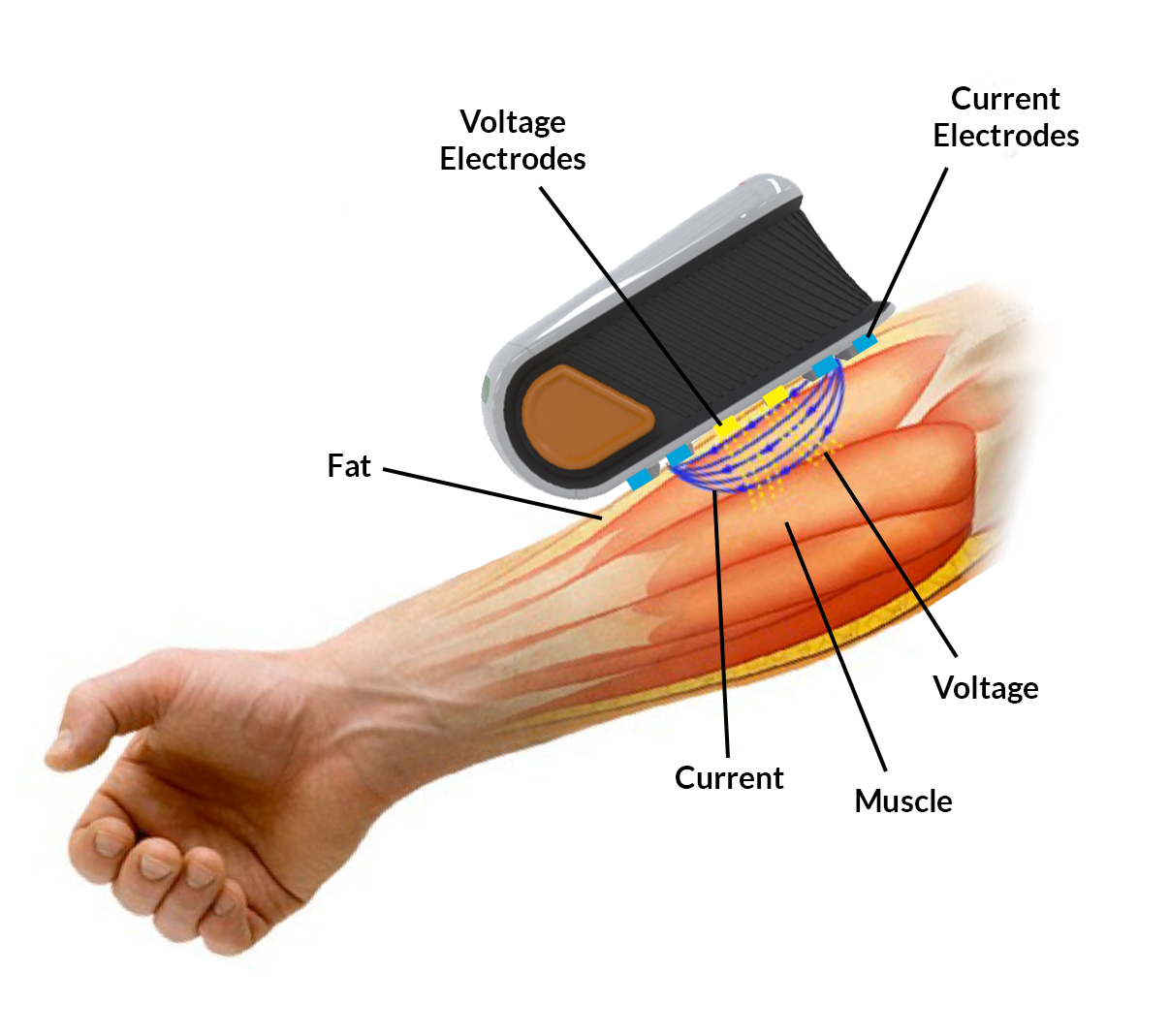
EIM comprehensively measures alterations in electrical impedance that occur due to disease.
PAINLESS
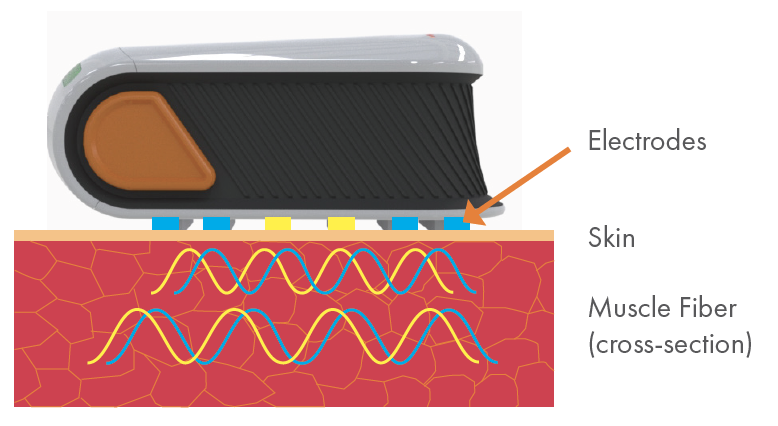
Imperceptible, high-frequency electrical current passes through muscle across 4 blue electrodes.
Resulting voltage signals are measured across 2 yellow sense electrodes.
Smaller, Standardized Clinical Trials?
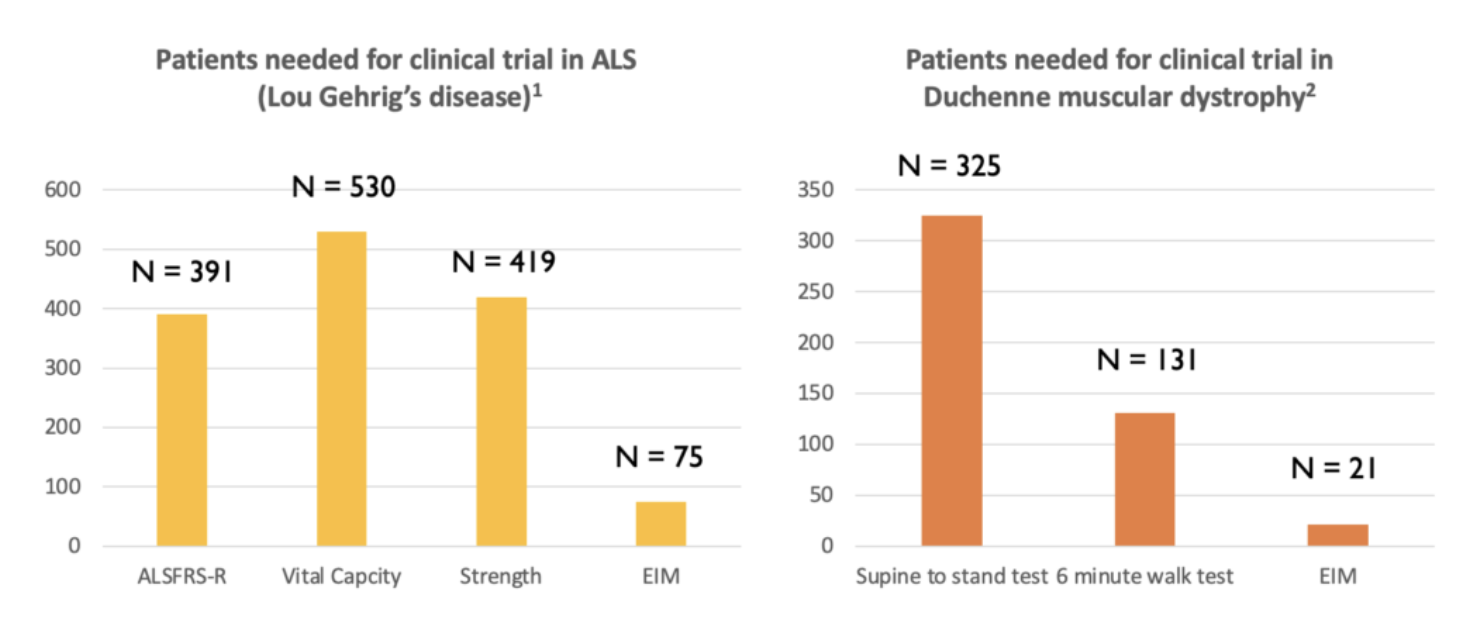
Myolex EIM has the potential to serve as a powerful, future biomarker that will be able to reduce clinical trial sample size requirements dramatically. Further, given it is non-invasive, portable, and easy-to-use, EIM enables standardized muscle condition data collection across all clinical trial sites.
Peer-reviewed studies have shown that the application of EIM to monitor ALS (Ref. 3) and DMD (Ref. 4) patients enables fewer patients to be enrolled in studies, as compared to the monitoring methods currently in use.
Possible.
Myolex EIM. The First of Its Kind.
Available Today for Clinical Research
Extensive Clinical Research

Let’s Discover Together.
1309 Beacon Street, Suite 300
Brookline, MA 02446
Phone: 888.382.8824
info@myolex.com





